Carbohydrate recognition by the type 1 pilus adhesion FimH
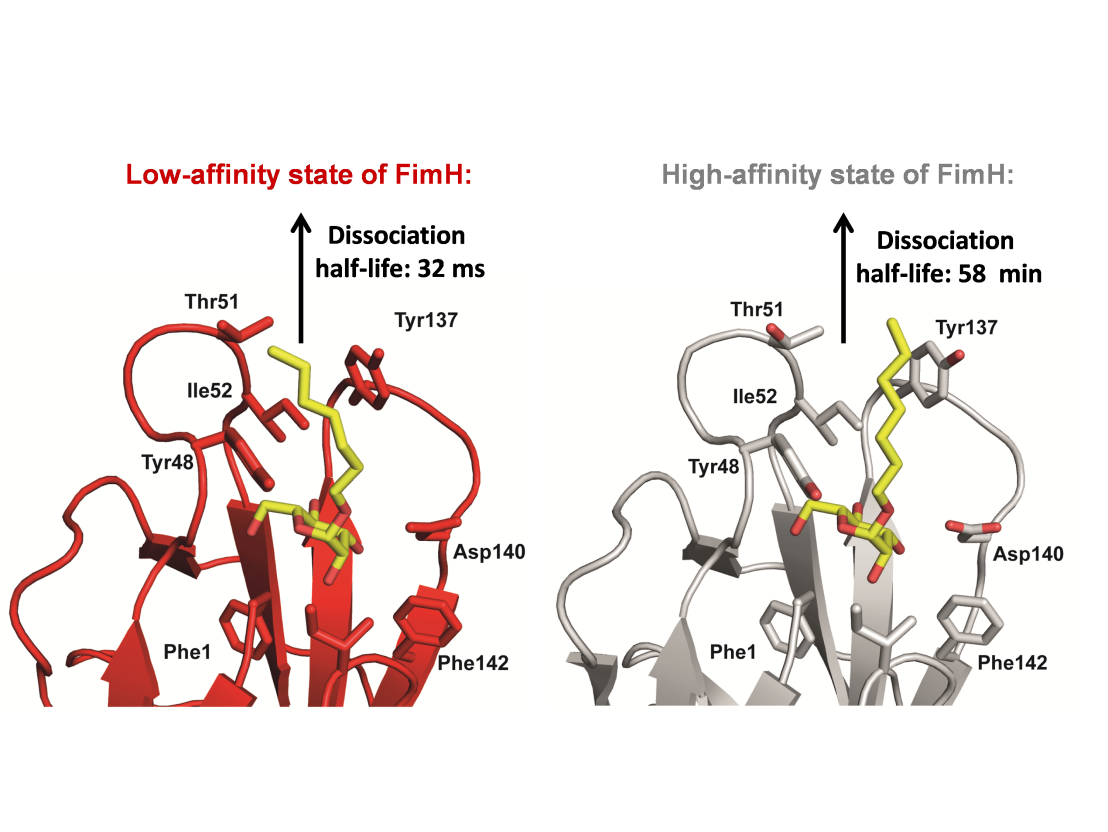
An important initial event in the establishment of a bacterial infection is the attachment of the bacterial pathogen to the host tissue. In the case of uropathonegic Escherichia coli strains, which are responsible for more than 80% of all urinary tract infections in humans, this adhesion step is mediated by the lectin FimH, the most distal subunit at the tip of filamentous type 1 pili. FimH recognizes terminal mannoses of uroplacin 1a, an abundant high-mannose type glycoprotein of urinary epithelium cells. FimH has the remarkable ability of forming “catch bonds” with its target ligands that increase the affinity of FimH for receptor molecules up to 3000-fold under the tensile mechanical forces occurring during urine excretion. In the absence of mechanical forces, the low affinity state of FimH allows the release of the bacteria from the urinary epithelium and bacterial invasion of new tissue areas. We demonstrated that the catch-bond mechanism of FimH is based on an allosteric mechanism in which the separation of its pilin domain from its lectin domain under shear forces induces a conformational change in the FimH lectin domain opposite to its carbohydrate binding pocket that decreases the rate of spontaneous dissociation of carbohydrate ligands from FimH by more than 100’000-fold.
Our current research goals are the following:
- Quantitative analysis of the thermodynamic linkage between domain separation and ligand binding affinity of FimH.
- Determination of the specificity of FimH for the three different terminal mannoses in high-mannose-type, N-linked glycans (a2-, a3- and a6-linked mannoses).
- Stoichiometry of FimH binding to high-mannose-type glycans.
- Interaction between FimH and uromodulin. Uromodulin is the most abundant protein in the human urine and is supposed to prevent urinary tract infections by competing with urinary epithelium cells for binding to FimH.
- Development of a protocol for determining the high-mannose type glycoproteome of eukaryotic cells using the high-affinity state of FimH as affinity matrix.