Open Positions

PhD open positions
Currently, there are no open PhD positions available.
Open projects
Structure and function of bacterial SPFH domain-containing proteins
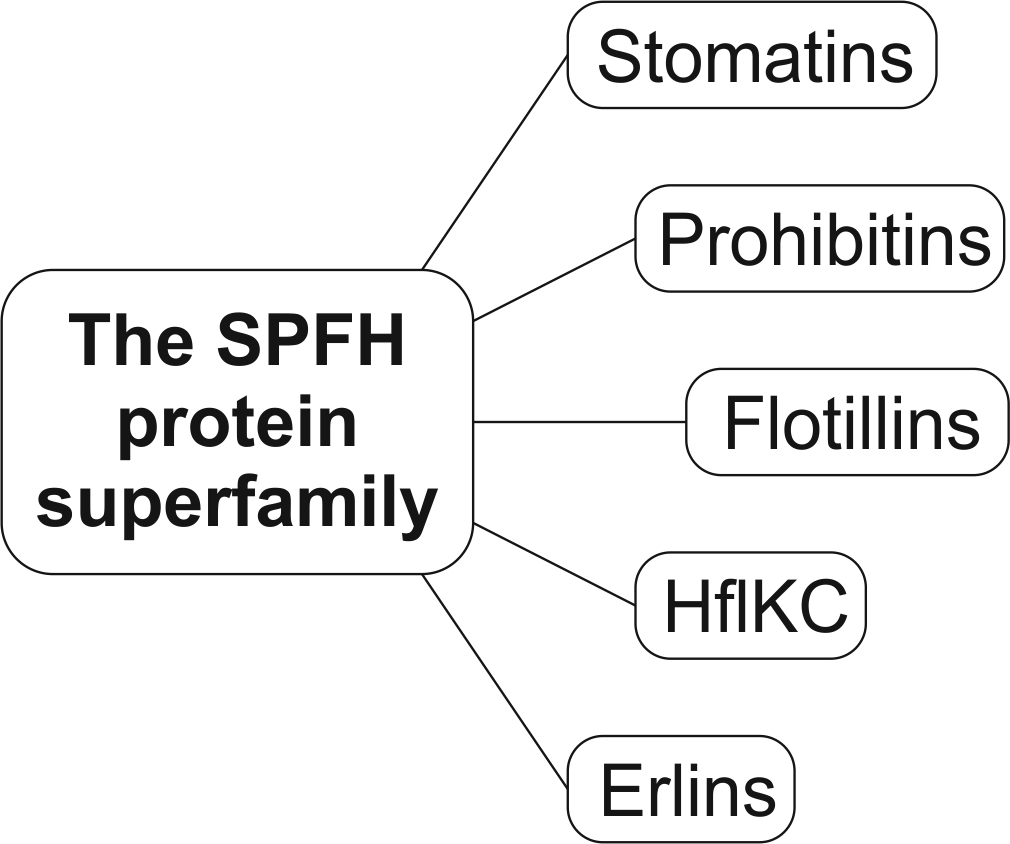
The SPFH proteins form a superfamily that is composed of five subfamilies: the slipins, prohibitins, flotillins, HflKC, and the more recently discovered erlins. All superfamily members share an evolutionary conserved, so-called SPFH domain, assemble into homo- or heterooligomeric multiprotein complexes and are membrane anchored. SPFH proteins are ubiquitous and occur in all domains of life. Consequently, they are involved in many central cellular processes such as for example regulation of protein degradation or modulation of ion channel activity, and their importance is further emphasized by the fact that prohibitins are essential in higher eukaryotes.
Despite their importance in the cell, the exact mechanisms of action of SPFH proteins are unclear. With this project, we aim to better understand structure and function of bacterial SPFH proteins, their molecular and mechanistic details, assembly and interaction with other proteins, and in particular the precise role played by the SPFH domain in these processes.
Biochemical, biophysical, genetic and structural techniques will be used to address these questions. For details, please contact Dr. Christoph Giese or Prof. Dr. Rudi Glockshuber - we are looking forward to hearing from you!
Bacterial pili as alignment medium for NMR spectroscopy
Type 1 pili of E. coli are filamentous, supramolecular protein complexes anchored in the outer membrane of the cell and extending into the extracellular space. They are composed of five different protein subunits called FimH, FimG, FimF, FimA and FimI. FimA is the main structural subunit of the pilus and up to several thousand FimA molecules form a right-handed helical quaternary structure, the so-called pilus rod, which has a diameter of 7 nm and is up to 2 µm long. Remarkably, the pilus is an extremely stable protein polymer with practically infinite stability against spontaneous dissociation and unfolding under physiological conditions. This unique feature makes the pilus a very attractive system for biotechnical applications.
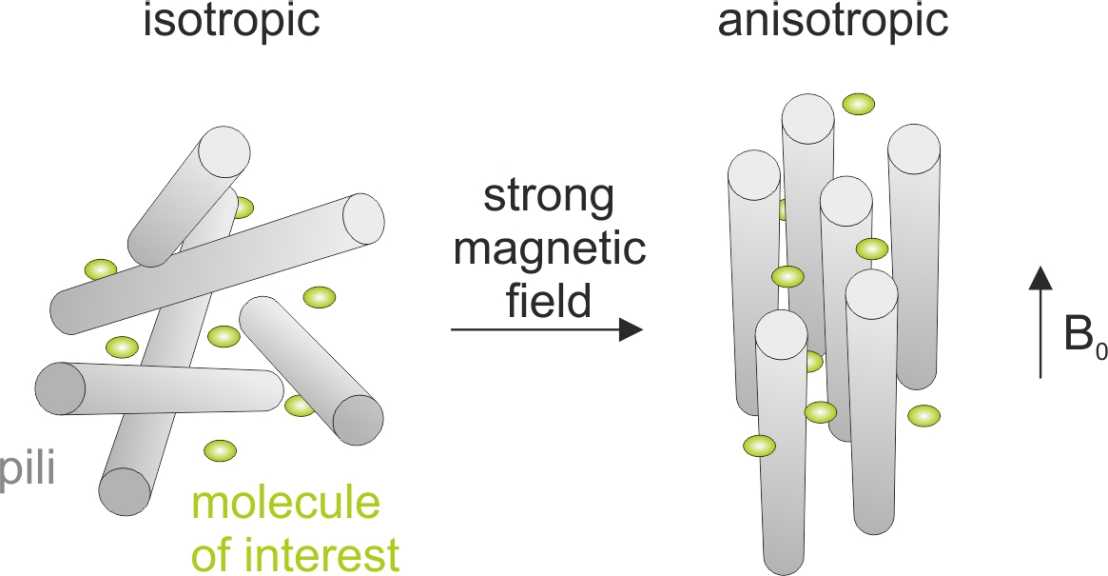
In collaboration with the group of Prof. Fred Allain at ETH we recently discovered that purified pili can generate anisotropic solvent conditions and weakly align biomolecules for measuring residual dipolar couplings in NMR spectroscopy.
The goal of the project will be to i) genetically and chemically engineer the wild-type pilus to modify its surface charge distribution, ii) investigate whether these pilus variants have different alignment properties and iii) characterize the pilus variants spectroscopically, structurally and with respect to their kinetic stability against unfolding and dissociation.
You will use molecular biology techniques, recombinant protein expression and purification, optical spectroscopy, electron microscopy and have the opportunity to get in touch with NMR spectroscopy. We are very much looking forward to welcoming you in our group, please contact Dr. Christoph Giese or Prof. Dr. Rudi Glockshuber for further details.
Master project on the mechanism of bacterial pilus biogenesis
We are looking for a semester student / MSc student interested in studying the assembly of supramolecular protein complexes using filamentous type 1 pili from uropathogenic Escherichia coli strains as a model system. Type 1 pili mediate bacterial attachment to epithelium cells of the urinary tract and thus play an important role in the establishment of infection. The type 1 pilus rod is a filamenotous homopolymer formed by several thousand copies of the pilus subunit FimA. In the pilus, the FimA subunits interact with a mechanism termed “donor strand complementation”, in which the incomplete, immunoglobulin-like fold of FimA is completed by an N-terminal extension of the following FimA subunit.
Using engineered variants of FimA, we have recently discovered an alternative mechanism of pilus rod formation in which inter-molecular donor strand complementation is eliminated, suggesting that the role of donor strand complementation in stabilizing the quaternary structure of the pilus has been overestimated. In contrast to wild type pili, the engineered pili can also be used for various technical applications.
The aim of the project is to compare wild type and engineered pili with respect to their three-dimensional structure, stability against dissociation and unfolding, and kinetic mechanism of polymerization, and to explore the use of the engineered pili as assembly platform for functionally improved multienzyme complexes.
Experimental techniques include molecular cloning, protein expression and purification in E. coli, negative stain electron microscopy, fluorescence and circular dichroism spectroscopy, reaction kinetics and X-ray crystallography.
For more information, please contact Dr. Dawid Zyla or Prof. Dr. Rudi Glockshuber